A double-blind study researched the weight-reducing efficacy of chitosan over a two to four week period. The study was performed at the ARS Medicina in Helsinki, Finland from mid-August to October of 1994.
| Changes of the
Systolic Blood Pressure Body Weight in KG. Group Averages 1 KG = 2.2 pounds |
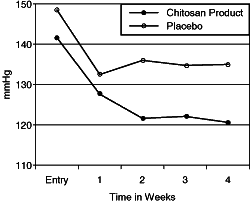 |
The primary objective of this study was to measure the weight reduction efficacy of the
chitosan in Chitosan Plus, but, secondarily, the researchers also wanted to look at the
effect on elevated blood pressure (hypertension).
Each patient took only eight 240 milligram capsules compared to a placebo. A special diet
for 14 days was presented to the two groups of patients. A dietitian from the Central
University Hospital of Helsinki developed the diet. It consisted of a typical western
composition: 40% of the calories as fat, 40% of the calories as carbohydrates, and 20% as
protein. The amount of calories, however, was restricted in both groups to 1,000 daily.
Any type of fiber was purposely left out of the diet so as to measure the true effect of
the chitosan.
| Changes of the
Diastolic Blood Pressure Group Averages |
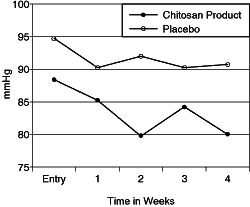 |
The initial length of the study was designed for 14 days, but some of the patients
decided to continue and were monitored for a longer period of time. The doctors
controlling the study performed weekly controlled visits. At each visit, the patients were
monitored for strict dietary control and to ensure they had been taking the chitosan
capsules as prescribed. The weight and blood pressure parameters were measured at each
visit. Each patient was to take 4 capsules of of chitosan twice daily, 4 before lunch and
4 before dinner each day. Each patient was cleared by medical examination in order to
participate in this study. Thirty patients started the study, but six withdrew and four
others were found to not be sufficiently overweight to participate in this study. Of the
remaining 20 patients, all but two fulfilled the four-week study period, eight were placed
in the active group, and ten in the placebo group.
| Reduction Body
Weight Body Weight in KG. Group Averages (1 KG = 2.2 pounds) |
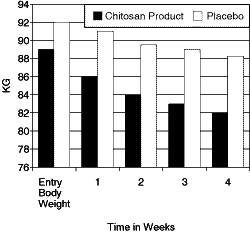 |
Both groups showed hypertension in their members, and both groups had a homogeneity
matched for body weight, height and body mass index. The placebo group members were an
average of six years younger in age, while the chitosan group members had an average age
of 50. If anything, this would help the placebo group in terms of superior response in
terms of losing weight. However, as we shall see, the results were significantly in favor
of chitosan.
Jan Abelin, M.D. and Allan Lassus, M.D. conducted the study. The average weight loss for
the chitosan group was a full 15 pounds in a 4-week period, whereas the placebo group only
had a weight loss average of 5 pounds. The weight reduction was also associated with a
significant decrease in blood pressure. The average diastolic blood pressure reading fell
20 millimeters of mercury with an additional 9 millimeters of mercury pressure drop in the
systolic.
It is a known fact that being overweight is a contributor to high blood pressure, which in
turn may lead to stroke. Here we have a single product that reduces body weight and lowers
the blood pressure as effectively as most drugs with no side effects whatsoever. The
researchers concluded that in this trial, chitosan appeared to be a nontoxic,
well-tolerated, effective natural product in helping people achieve weight reduction and
normal blood pressure.